On June 13, 2019, the research team led by Dr. Linqi Zhang at School of Medicine of Tsinghua University reported on identification and characterization of a panel of HIV-1 strains with broad and potent resistance against a large number of broadly neutralizing antibodies (bnAbs), particularly those targeting the CD4-binding site (CD4bs). Resistance is largely attributed to mutated residues within the epitopes or steric hindrance imposed by the bulky side-chain or glycan shield of the mutated residues, and is largely correlated with reduced binding avidity of the antibody to the quaternary trimeric envelope protein expressed on the surface of the transfected cells. Treatment strategies must overcome such resistance to achieve optimal clinical outcomes. The research work has just been reported in a prestigious journal PLOS Pathogen.
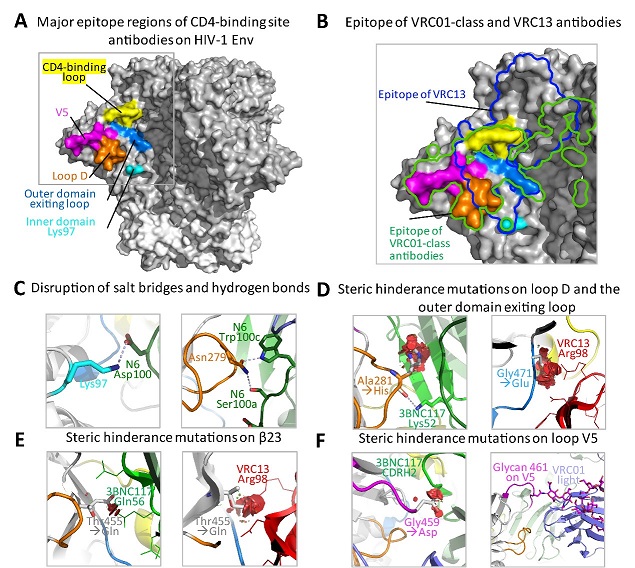
Figure 1. Structural basis of the signature resistance mutations
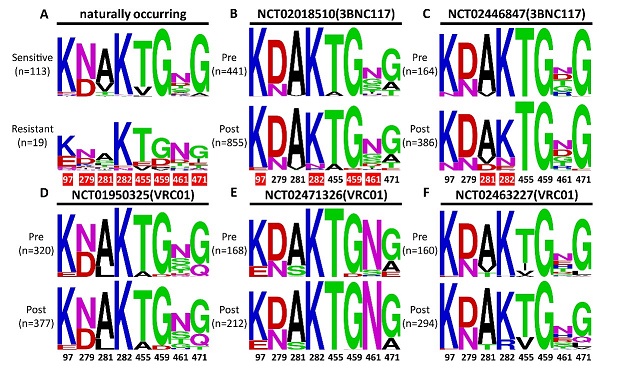
Figure 2. Polymorphisms and shifts at the signature residues before and after antibody treatment
AIDS is a major global infectious diseases that threaten human health. Since its discovery in 1981, it has been spread rapidly around the world. By the end of 2018, approximately 77.3 million people had been infected. Of which, about 35.4 million had died. Despite tremendous efforts made by the scientists worldwide, there are still no effective vaccines. Human Immunodeficiency virus type I (HIV-1) is the culprit of AIDS. It mainly infects and kills critical immune cells such as CD4+ T cells, leading to series of opportunistic infections and tumors and ultimately death. Comparing to other viruses, HIV-1 is extremely rapid in generating and turning over of viral variants. In recent years, increasing number of bnAbs isolated from elite controllers have demonstrated impressive potency and breadth against HIV-1 both in vitro and in vivo. These antibodies are capable of neutralizing more than 90% of HIV-1 circulating around the world and have great potential in therapeutic and prophylactic interventions against HIV-1 infection. Broadly speaking, these bnAbs recognize six major “vulnerable sites” on the HIV-1 envelope glycoprotein gp160 (gp120 and gp41), which are (1) the CD4-binding site (CD4bs), (2) the V1V2 apex, (3) the V3 glycan, (4) the fusion peptide (FP), (5) subunit interface and (6) membrane proximal external region (MPER) of gp41. Among the bnAbs isolated to date, those targeting the CD4bs are the most abundant and thoroughly studied as they disrupt the first and crucial step of viral interaction with the cellular receptor CD4. Some of the CD4bs bnAbs have demonstrated encouraging results in initial clinical trials. However, the discovery of broadly resistant HIV-1 virus reveals that the virus can be highly mutable during natural infection and poses a big challenge for the clinical use of these bnAbs. More potent and broadly effective antibodies are needed, either by isolating antibodies with exceptional neutralizing activity from patients, or engineering the existing antibodies into bi- or tri-specific combinations
The research was led by Dr. Linqi Zhang at Tsinghua University, in close collaboration with Dr. Tongqing Zhou from Vaccine Research Center (VRC) at the National Institutes of Health (NIH) and Dr. Hong Shang from No. 1 Hospital of China Medical University. Panpan Zhou from Dr. Zhang’s laboratory is the leading author, and Linqi Zhang and Tongqing Zhou share the co-corresponding authors. The research was supported by the National Natural Science Foundation Award, the National Science and Technology Major Projects, Beijing Municipal Science and Technology Commission, Ministry of Science and Technology of China and Gates Foundation Grand Challenges China.
Article link: https://doi.org/10.1371/journal.ppat.1007819