Professor Du Yanan and His Team’s Innovative 3D Stem Cell Technology Supports the Approval of China’s First Stem Cell therapy, Amimestrocel Injection
On January 2, 2025,the first stem cell-therapy in China, amimestrocel injection, developed by Platinum Life Excellence Biotech (Beijing) Co., Ltd., has been granted conditional market approval for the treatment of steroid-refractory acute graft-versus-host disease (aGVHD). This new mesenchymal stem cells (MSCs) therapy offers a promising treatment option for patients aged 14 and older, particularly those with gastrointestinal complications who have failed conventional steroid treatments.
As China’s first stem cell therapy, Amimestrocel Injection utilizes cutting-edge technology developed by Professor Du Yanan’s team at the School of Biomedical Engineering, Tsinghua University. The team’s innovative 3D microcarrier-based stem cell culture technology has strongly supported the drug’s production process. This technology allows for large-scale, automated, closed, and continuous stem cell manufacturing, upgrading traditional manual cell culture methods to a modern “cell factory.”
The innovative cell manufacturing technology offers a more natural 3D environment for stem cell growth while effectively reducing contamination risks associated with open operations. It also significantly lowers material, labor, and time costs in the manufacturing process, ensuring that stem cells meet the rigorous quality and safety standards required for clinical applications. The technique simultaneously meets the needs of high-quality stem cell production and large-scale commercial manufacturing.
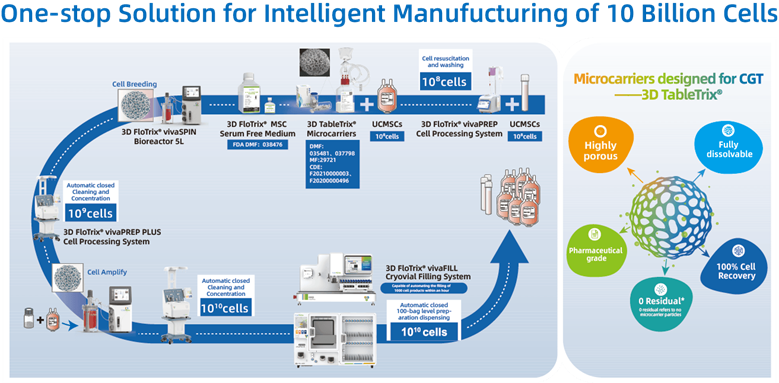
Automated, closed, large-scale stem cell manufacturing process based on 3D microcarriers.
Previously stem cell therapy, either approved in for foreign countries or under clinical trials in China was primarily based on two-dimensional plastic surfaces and manual cell culture processes. These methods were plagued by high labor costs, large space requirements, limited batch production, and inconsistent product quality. Professor Du Yanan’s team has spent over a decade developing large-scale 3D cell manufacturing technology, which played a key role in the successful launch of China’s first stem cell drug. Amimestrocel Injection’s approval marks the first 3D microcarrier-based stem cell therapy entering clinical use.
"Life and diseases are highly complex. We must choose the appropriate types of stem cells for different indications and place a strong emphasis on the scientific and compliant application of stem cell therapy to minimize potential risks." Professor Du emphasized the importance of selecting appropriate stem cells for different indications and ensuring the scientific and compliant application of stem cell therapies to mitigate potential risks.
Professor Du’s research team has long been focused on innovations in microtissue engineering technologies and their translation into clinical applications, contributing to the advancement of stem cell-based medicine in China and globally.
In addition to mesenchymal stem cells, Professor Du’s team has developed 3D culture technologies for the industrial-scale preparation of hematopoietic stem and progenitor cells, as well as pluripotent stem cells, providing potential solutions for the industrialization and clinical use of other types of stem cell therapies.