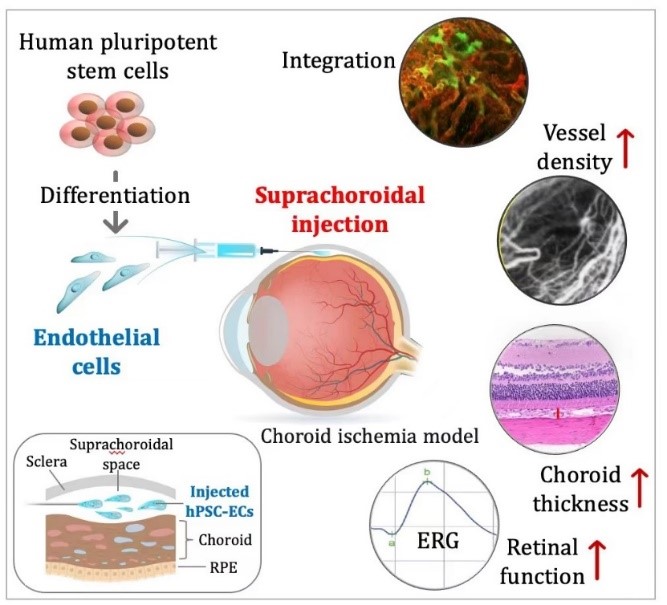
The choroid supplies nutrition and oxygen for the retinal pigment epithelial cells, the outer five layers of the retina, especially in the macular fovea. Choroidal capillary atrophy is a significant pathological change found in many blinding eye diseases such as age-related macular degeneration, retinitis pigmentosa, myopic maculopathy, etc. However, currently there is no effective treatment. Transplantation of choroidal endothelial cells from stem cell sources provided a new strategy to treat choroidal ischemia.
In December 2023, a joint team of Professor Hu Yuntao's group from the Department of Ophthalmology at Beijing Tsinghua Changgung Hospital and Associate Professor Na Jie's laboratory from the Center for Stem Cell Biology and Regenerative Medicine at Tsinghua University School of Medicine published a research paper “Human pluripotent stem cells derived endothelial cells repair choroidal ischemia” in 'Advanced Science' (IF 17.521). They reported the therapeutic effects of human pluripotent stem cells derived choroidal-like endothelial cells (hPSC-CEC) on choroidal ischemia.

Researchers first differentiated hPSCs into choroidal endothelial cells which expressed specific choroid markers CD31, CA4, RGCC, PV-1, had porous structures on the cell membrane and typical endothelial physiological functions (Figure 1).
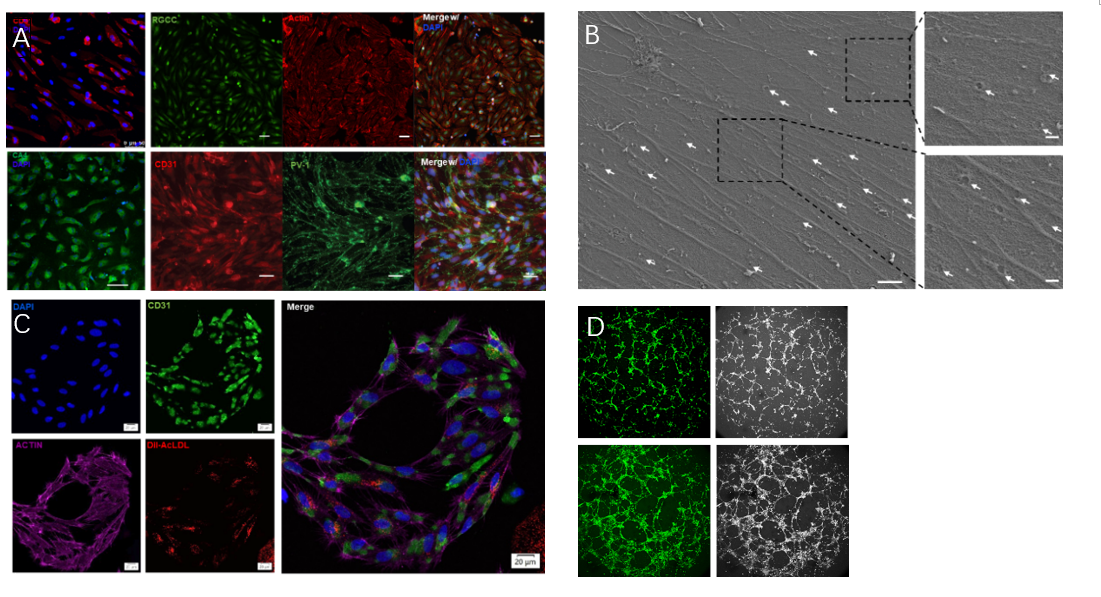
Figure 1. Choroidal vascular endothelial cells derived from human pluripotent stem cells. A. Expression of specific markers for choroidal endothelial cells (CD31, CA4, RGCC, PV-1); B. Fenestration-like structures on hPSC-CECs which typical of choriocapillary cells (white arrows); C. Endothelial cells exhibited lipid droplet engulfment function; D. Tubular formation by endothelial cells.
The researcher team subsequently established a rat model of choroidal ischemia and transplanted hPSC-CECs into the suprachoroidal space. Amazingly, hPSC-CECs integrated into the rat choroidal vasculature efficiently and increased choroidal thickness. Indocyanine green (ICG) choroidal angiography revealed recovery of the vascular network in the ischemic area. Electroretinography (ERG) confirmed a significant improvement in visual function in choroidal ischemic rats following hPSC-CEC transplantation (Figure 2).
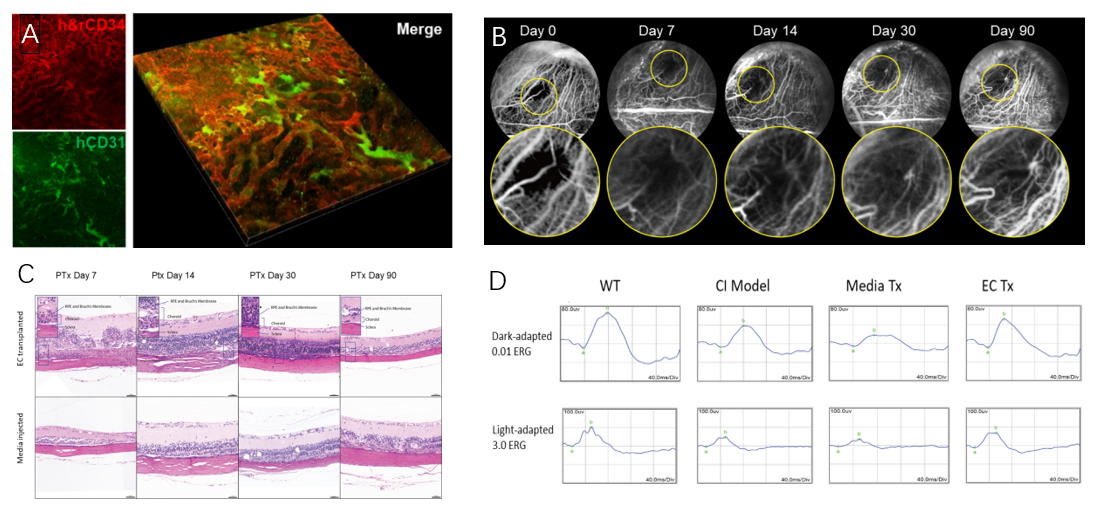
Figure 2. The reparative effect of hPSC-CECs on rat choroidal vasculature. A. hPSC-CECs integrate into the rat's own vasculature in the choroidal ischemic area and promote vascular regeneration (CD34 labeling rat and human endothelial cells; CD31 labeling human endothelial cells); B. Indocyanine green (ICG) angiography of rat fundus demonstrates the formation of a new vascular network in the choroidal ischemic area after transplantation; C. Increased choroidal thickness post-cell transplantation; D. Electroretinography (ERG) in rats indicates a significant improvement in retinal function after cell transplantation.
Interestingly, upon co-culture with rat ischemic choroid, hPSC-CECs upregulated multiple genes known to promote endothelial cell migration, vascular generation and protection, immune modulation, retinal repair, and inhibit pathological neovascularization (CNV), which may explain their superior reparatory effect in vivo (Figure 3).
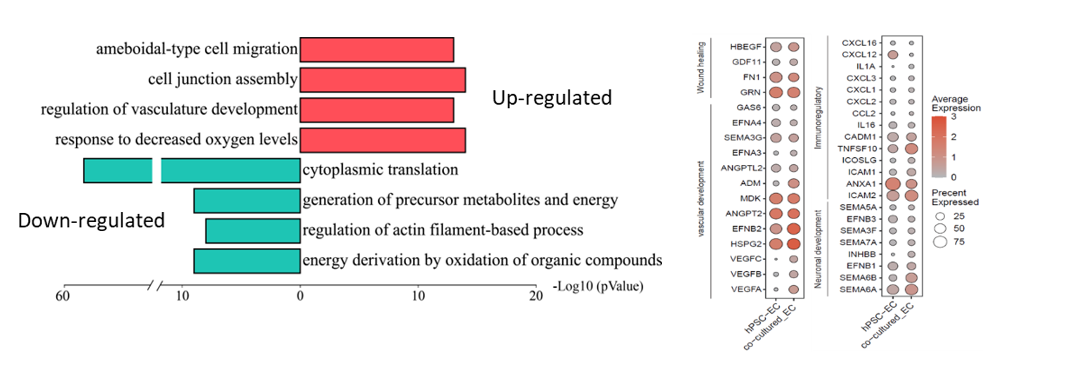
Figure 3. Single-cell RNA sequencing (scRNA-seq) analysis revealed that hPSC-CECs upregulated genes associated with vascular generation, immune modulation, and neuroprotection when co-cultured with ischemic choroid.
In summary, this study characterized hPSC-CECs and transplanted them into the suprachoroidal space of an animal model of choroidal ischemia, which effectively ameliorated choroidal ischemia and improved visual function. The study opens a new venue for treating choroidal ischemia-related diseases, which has significant potential for clinical application.
Professor Hu Yuntao from the Department of Ophthalmology, Beijing Tsinghua Changgung Hospital and Associate Professor Na Jie from Tsinghua University School of Medicine are the corresponding authors. Dr. Li Mengda, attending physician at Beijing Tsinghua Changhung Hospital's Department of Ophthalmology, Huo Si Tong (graduated MSc), Ph.D. candidate Wang Peiliang, and Qiu Hui are the co-first authors. Dr. Lin Siyong, Chief Physician at the Department of Ophthalmology, Beijing Tsinghua Changgung Hospital, Associate Chief Physician Guo Libin, Dr. Li Chendi, Technician Ji Yicong, Dr. Zhu Yonglin (graduated) from Tsinghua University School of Medicine, Ph.D. candidate Liu Jinyang, and Dr. Guo Jianying, Research Associate at the Department of Obstetrics and Gynecology, Peking University Third Hospital, made important contributions to this study. Professor Wong Tien-Yin, Vice Dean of Tsinghua University, a renowned ophthalmologist, gave valuable suggestions for this research.
This study was supported by grants from the National Key Research and Development Program of China, the National Natural Science Foundation of China, Tsinghua University's Spring Breeze Fund, Tsinghua University's Precision Medicine Research Program, and funding from the Shanxi Medical University-Tsinghua University Joint Research Center for Frontier Medicine.
Weblink:http://doi.org/10.1002/advs.202302940