Breast cancer is one of the leading causes of cancer-related mortality in women.Immunotherapy, especially immune checkpoint inhibitors (ICIs), including PD-1, PD-L1 and CTLA-4 blocking antibodies, has generated remarkable responses in many individuals with solid tumors. However, in breast cancer, especially triple-negative breast cancer (TNBC), limited patients benefit from ICI therapy, indicating that there are other immunosuppressive molecules in the tumor microenvironment of breast cancer, limiting the efficacy of immunotherapy.Exploring the new molecular mechanisms of immune evasion in breast cancer will identify new therapeutic targets and help resolve the ICI resistance.
Jagged1, one of the major Notch ligands, is overexpressed in about 30% of individuals with breast cancer and its overexpression is associated with poor prognosis. However, the role of Jagged1 in breast tumorigenesisand whether it is involved in immune evasion is not clear.Prof. Hanqiu Zheng from Tsinghua University School of Medicine, in collaboration with Professor Yibin Kang of Princeton University, Professor Zhi-Ming Shao andProfessor Yi-zhou Jiang of Fudan University, discovered that Jagged1 promotes tumor progression by modulating the adaptive immune microenvironment.
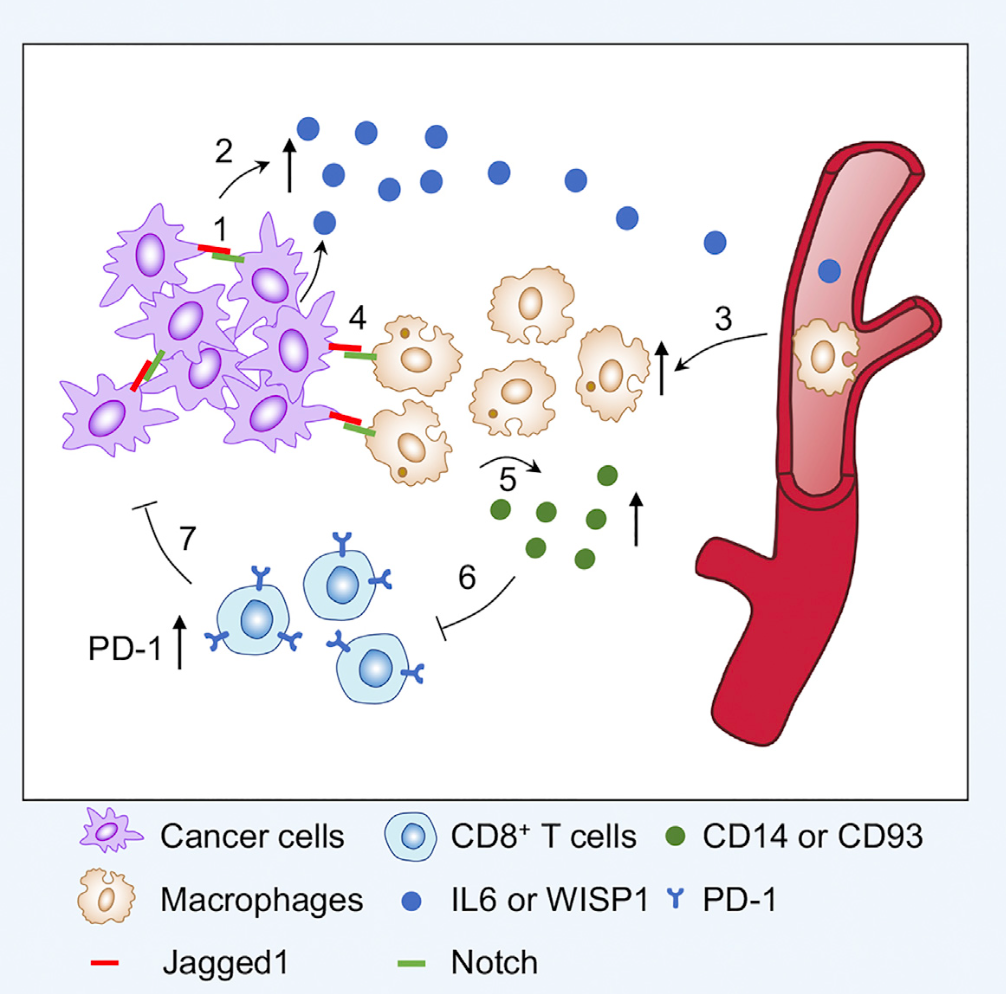
Figure 1:Jagged1 promotes immune evasion to drive tumor growth
The research team generated tissue-specific Jagged1 gain-of-function and loss-of-function mouse strains. They also crossed these strains witth spontaneous mouse mammary tumor models.Jagged1 overexpression promotes tumorigenesis in these spontaneous mouse models, whereas Jagged1 knock-out in mammary gland leads to a significant delay of tumor onset and reduced tumor growth rate.Jagged1 also promotes lung metastasis as loss of Jagged1 reduced lung metastatic nodules in these mice.Immune cell profiling identifies increased macrophage infiltration and decreased T cell presentation in Jagged1 overexpression tumors. By utilizing multiple state-of-the-art assays, researchers found that through Jagged1-induced Notch activation, tumor cells increase the expression and secretion of multiple cytokines, including IL6 and WISP1, to help recruit macrophages into the tumor microenvironment. "Educated" macrophages crosstalk with tumor-infiltrating T cells to inhibit T cell proliferation and tumor-killing activity.Preclinical mouse experiments show that the co-administration of PD-1 antibody with a Notch inhibitor significantly suppresses tumor growth in several tumor models, suggesting that this might be a new combinational immunotherapy for breast cancer patients.
This study proves that Jagged1 promotes immune escape to drive tumor growth, and can be used as a potential immunotherapy target, either alone or in combination with PD-1 blocking antibody therapy. The research team has long been committed to the study of tumor microenvironment and drug resistance. Combined with their previous studies (Sethi et al., 2011; Zheng et al., 2017), their results suggest that Jagged1 utilizes a different set of stromal cells to promote primary tumor progression and bone metastasis. During bone metastasis, tumor cells engage bone-residential osteoclasts andosteoblasts to enhance osteolytic bone metastasis and chemoresistance; at the primary site, tumor cells interact with tumor-associated macrophages to suppress tumor-killing T cells.Jagged1 might therefore belong to a potential new set of cancer driver genes that it interacts with stromal cells differentially at primary site and at metastatic site, but contributes to multiple steps during tumor prgression, including tumor initiation, immune evasion, metastasis, and therapy-resistance. Small molecule inhibitors or blocking antibodies against Jagged1 could be clinically developed in the future to benefit the patients.
"Tumor-derived Jagged1 promotes cancer progression through immune evasion" by Menget al.appears in the March 9th issue ofCell Reports.
Hanqiu Zheng, Associate Professor, School of Medicine, Tsinghua University is the lead contact of this paper. Prof. Yibin Kang of Princeton University and Prof. Zhi-ming Shao of Fudan University Cancer Hospital are the co-corresponding authors of this article. Jingjing Meng, a PhD student of 2016 from the Tsinghua-Peking Center for Life Sciences, is the first author of this article. This project was funded by National Key Research and Development Program of China, the National Science Foundation of China, and the Tsinghua-Peking Center for Life Sciences
Original paper link:
https://www.cell.com/cell-reports/fulltext/S2211-1247(22)00225-X
Reference
1. Sethi et al., Tumor-Derived Jagged1 Promotes Osteolytic Bone Metastasis of Breast Cancer by Engaging Notch Signaling in Bone Cells. Cancer Cell (2011) 19, 192-205.
2. Zheng, H. et al., Therapeutic Antibody Targeting Tumor- and Osteoblastic Niche-Derived Jagged1 Sensitizes Bone Metastasis to Chemotherapy. Cancer Cell (2017) 32, 731-747.e736.
3. Meng et al., Tumor-derived Jagged1 promotes cancer progression through immune evasion, Cell Reports (2022), https://doi.org/10.1016/j.celrep.2022.110492