Hematopoietic stem cells (HSC) reside in the bone marrow and possess the ability to self-renewal and differentiate into all blood cell types; therefore hold great potential in cell-based therapies and regenerative medicine. In 2019, Zeng et al. reported the single-cell map of hematopoietic stem cells (HSCs) generated in the human embryo (Zeng et al., 2019). However, due to the inaccessibility of the early human embryo and minimal cell number, the epigenetic landscape, including genome-wide distribution of open chromatin and key histone modifications during HSC development, remains elusive. Human pluripotent stem cells (hPSCs) differentiation towards hematopoietic stem/progenitor cells (HSPCs) provides an in vitro experimental system to investigate the epigenetic regulation of human embryonic hematopoiesis.
On June 6, 2022, Dr. Jie Na’s group published a paper entitled "Integrative epigenomic and transcriptomic analysis reveals the requirement of JUNB for hematopoietic fate induction" in Nature Communications. In this study, researchers systematically profiled the genome-wide chromatin accessibility, histone H3K4me3, and H3K27me3 modifications and gene expression during sequential stages of hematopoietic fate specification, as well as the single-cell transcriptome and accessible chromatin during the endothelial-to-hematopoietic transition (EHT) window. Analysis of the multi-omics datasets revealed sequential opening-up of chromatin sites for key transcription factors (TFs) regulating hematopoiesis and stage-specific change of genes with bivalent histone modification. ScRNA-seq data showed that hPSC-derived hemogenic endothelial cells (HEC) and HSPCs had reduced arterial features compared to their in vivo counterparts. After a combined analysis of the chromatin and gene expression feature, researchers identified JUNB as a critical TF for HEC specialization and the EHT process, which are pivotal to HSPC formation.
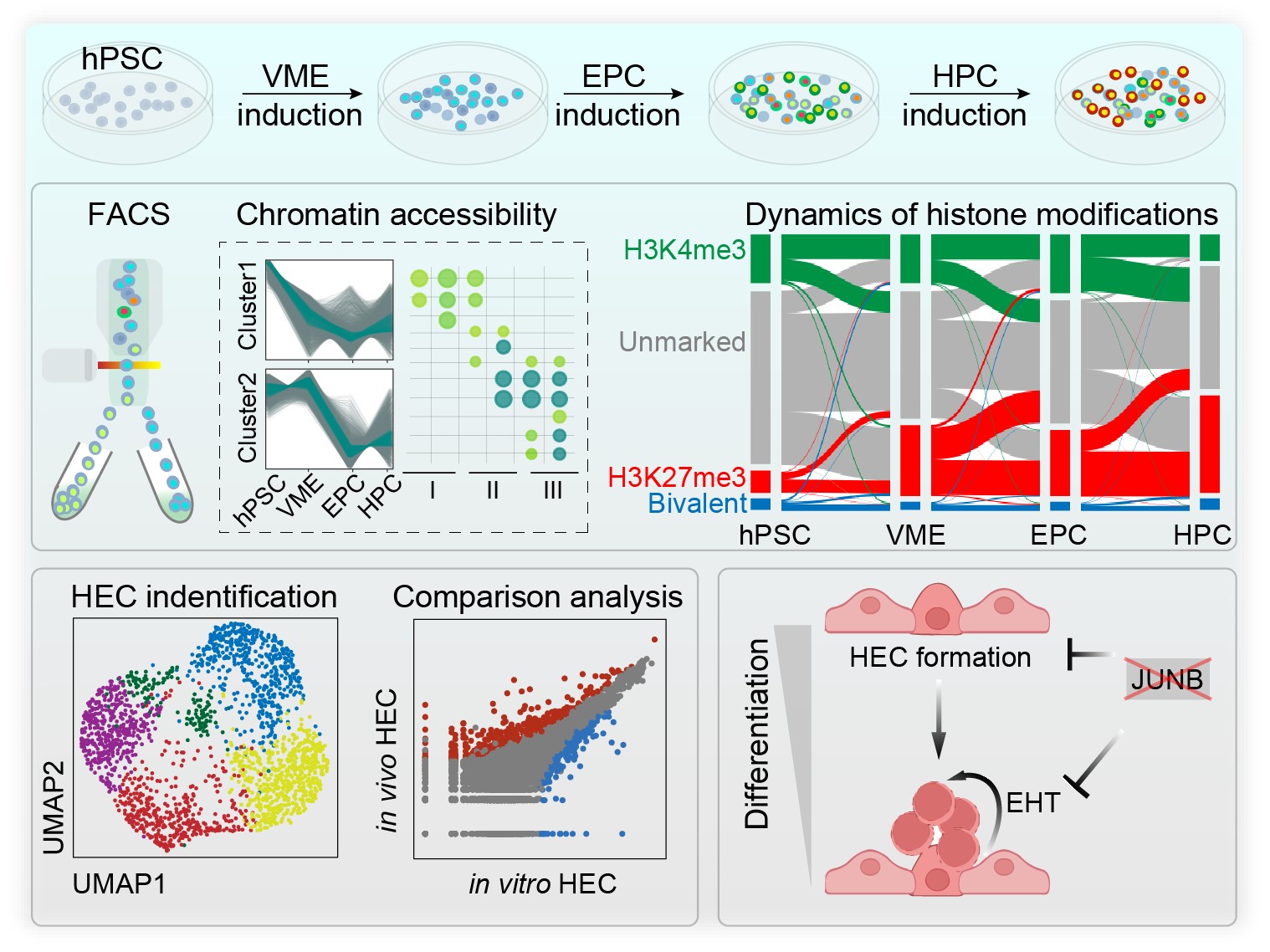
This study enriched the knowledge about the epigenetic regulation of hematopoietic specification in the human system and provided clues to optimize in vitro differentiation to obtain HECs and HPCs with more robust hemogenic potentials.
Dr. Jie Na from the School of Medicine, Tsinghua University, is the corresponding author of this study. Dr Xia Chen, Ph.D. candidates Peiliang Wang, and Hui Qiu are the co-first authors. This work was supported by the National Key R&D Program of China Grant 2019YFA0110001 and 2017YFA0102802, the National Natural Science Foundation of China grant 31970819、91740115、31771108、32000610, and The Tsinghua University Spring Breeze Fund 2021Z99CFY033.
The web link to the article:
https://www.nature.com/articles/s41467-022-30789-4
Zeng, Y., He, J., Bai, Z., Li, Z., Gong, Y., Liu, C., Ni, Y., Du, J., Ma, C., Bian, L., et al. (2019). Tracing the first hematopoietic stem cell generation in human embryo by single-cell RNA sequencing. Cell Res 29, 881-894. 10.1038/s41422-019-0228-6.